Which Of The Following Cooking Methods Would You Use To Increase The Digestibility Of Protein?
Introduction
Proteins are important components of the human diet and play an essential role as structural and functional components of living systems. Food proteins provide amino acids (AA) which serve as building blocks of all vital organs, muscles (including heart muscles), hormones and biological fluids such as blood. As the human body is incapable of maintaining reserves of protein, a constant supply of good quality protein is needed to maintain growth and other physiological functions. Insufficient intake of protein especially during periods of growth and development can affect all organs in the body including the brain, heart, immune system, and other vital organs. Protein quality of foods is, therefore, an important criterion for the provision of adequate nutrition and maintenance of good health.
A significant effort has been made in the last several decades to establish methods for evaluating protein quality. The 1957 FAO Committee report on Protein Requirements( 1 ) recommended the use of a reference protein with an "ideal" AA composition to define the pattern of human AA requirements. In 1963, a Joint FAO/WHO Expert Group( 2 ) further discussed the need to take into consideration the rate of obligatory nitrogen loss from the body on a protein-free diet when determining human protein requirements. Discrepancies between the obligatory nitrogen loss and the minimum nitrogen intake needed to ensure balance, were considered by an Ad Hoc Expert Committee convened in 1971( 3 ). The concern was that even for proteins with a high biological value, higher amounts of protein were required than that indicated by the obligatory nitrogen loss. The committee also recommended a safe level of intake for a population, which was defined as the average protein requirement of the individuals in the population plus 1·96 times the standard deviation (SD). A follow-up Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements in 1981( 4 ) emphasised the need to consider digestibility in the evaluation of protein quality. Eight years later, the Joint FAO/WHO Expert Consultation on Protein Quality Evaluation( 5 ) recommended the use of the Protein Digestibility Corrected Amino Acid Score (PDCAAS) method for protein quality evaluation. The PDCAAS method has now been in use for 20 years. The 2007 report of the FAO/WHO/UNU Expert Consultation on protein and AA requirements in human nutrition( 6 ) highlighted several aspects of protein quality that still required consideration. The report also provided new reference patterns based on AA requirements for different age groups.
The current paper provides a review of the use of the PDCAAS method compared to other methods for evaluating protein quality and addresses some of the key challenges that remain in regards to protein quality evaluation. A brief overview of human AA and protein requirements and factors influencing dietary protein quality is provided, as well as a summary of the progress made by the international science community to identify acceptable methods for evaluating protein quality. Current recommendations for protein intake are also reviewed and specific examples of standards and requirements used in a few selected countries vis-à-vis the WHO/FAO/UNU standards are presented.
Human amino acid and protein requirements
Proteins are made up of amino acids linked by peptide bonds and are the main supply of nitrogen in the diet. To sustain bodily functions and growth, humans require certain minimal levels of protein intake as well as adequate supplies of dietary essential amino acids that are not synthesized by the body. The aim of protein quality evaluation is to determine the ability of a protein to meet maintenance needs plus special needs for growth, pregnancy, or lactation( Reference Millward, Layman and Tomé 7 ). For most adults, protein intake should be in equilibrium with protein loss. Positive protein balance is, however, required for growing infants and children and during pregnancy and lactation, and requirements may also increase during times of illness and recovery.
Nitrogen balance in humans, which is a measure of daily nitrogen intake minus nitrogen excreted, is a reflection of both protein and energy intake from the diet. Protein nutritional requirement is, therefore, defined as the lowest level of dietary protein intake that will balance the losses of nitrogen from the body, and thus maintain the body protein mass (i.e., in persons at energy balance with modest levels of physical activity)( 6 , Reference Millward, Layman and Tomé 7 ). In children or pregnant/lactating women, this further includes needs associated with the deposition of tissues or the secretion of milk at rates consistent with good health.
Factors influencing dietary protein quality
Protein quality may be defined as the ability of a food protein to meet the body's metabolic demand for amino acids and N and is determined by the AA composition and digestibility of the protein as well as the bioavailability of the individual AA. The term is relative allowing a comparison of the adequacy of different sources of food proteins to meet human protein requirements. Both digestibility and bioavailability are affected by the food matrix (e.g., levels and types of fat, carbohydrate and antinutritional compounds). Other factors influencing protein quality are demands that are specific to the individual consuming the food, such as age, health status, physiological status and energy balance( Reference Millward, Layman and Tomé 7 ).
Amino acid composition
Amino acids are classified into those which cannot be synthesized by the body and therefore must be obtained by the diet, "indispensable" (IAA) or "essential" (EAA) (His, Ile, Leu, Lys, Met, Phe, Thr, Trp and Val), and those which the body can produce, "dispensable" or "nonessential" (Asp, Asn, Glu, Ala, Ser, Cys, Tyr, Gly, Arg, Gln, Pro). However, these two categories are not absolute or mutually exclusive: the endogenous capacity for the formation of some dispensable AA may not always meet demand and, potentially, some de novo synthesis of IAA may occur following urea salvage in the lower gut( 6 ). Some AA, such as Cys, Tyr, Gly, Arg, Gln, Pro and taurine, are termed "conditionally indispensable", as they become dietary essential only under specific pathological or physiological conditions.
The 2007 WHO/FAO/UN Expert Consultation on proteins( 6 ) stressed the need to interpret these classifications with care, as there appears to be an absolute metabolic need for both dispensable and indispensable AA. The efficiency of utilization of IAA is dependent on the total N and the form of N in the diet. The higher the dietary total N, the lower the amount of IAA needed to achieve N-balance( 6 ). The report further states that when all or any of the IAA are present in excess of demand, the absorbed mixture is unbalanced and limited by dispensable AA, which would need to be supplied from oxidation of surplus IAA. However, the biological value of a protein is defined in terms of how well the profile of IAA in a protein matches that of the pattern required by body. Some authors have argued that IAA are important at higher intakes than those in the requirement pattern, especially in the case of high quality proteins (e.g. egg, milk, fish and meat protein products) that are used to supplement other low quality proteins( Reference Sarwar 8 ). Another argument supporting higher intakes of IAA is that their role extends beyond that of supporting growth or N balance (i.e., implications in such diverse functions as lean body mass retention, cell signalling, bone health, glucose homeostasis and satiety induction)( Reference Millward, Layman and Tomé 7 ).
Protein digestibility
In order for constituent AA to be released, proteins must first be digested. A possible exception is in neonates where some uptake of intact proteins or peptides from the intestinal lumen into the systemic circulation can occur. Digestibility is usually defined in terms of the balance of AA across the small intestine (mouth to terminal ileum: ileal digestibility), or across the entire gut (mouth to anus: faecal digestibility), based on the principle that the difference between intake and losses provides a measure of the extent of digestion and absorption of food protein as AA by the gastrointestinal tract for use by the body( 6 ). This net balance across the digestive tract is a complex process that involves considerable exchange of nitrogen in terms of protein, AA and urea between systemic pools and the gut lumen( 6 ). Large differences can occur between the digestibility of a protein and specific AA, especially where antinutritional factors are present, such as in uncooked cereals and legumes( Reference Gilani and Sepehr 9 ).
The digestive process starts with hydration and solubilization in the mouth. Most food proteins remain intact until reaching the stomach. A range of different proteolytic enzymes (some specific to certain AA) are necessary to break the peptide bonds within the protein chain. HCl in the stomach denatures some proteins, making the peptide bonds more accessible for proteolysis by digestive enzymes. The HCl also converts the pepsinogen secreted by the stomach to the active protease pepsin, which cleaves the protein into peptide fragments. The pancreas secretes enzymes such as trypsin, chymotrypsin, carboxypeptidases, collagenase and elastase into the duodenum, and these enzymes break up the peptides into smaller peptide fragments. The final digestion occurs in the small intestine by enzymes such as aminopeptidases and tripeptidases, which split the remaining short peptide fragments into single AA or di- and tri-peptides, which can be absorbed by the intestinal mucosal cells. Undigested and unabsorbed nitrogenous residues are then transported to the large intestine (colon) where further microbial modifications of this material are possible within the large intestine, prior to faecal excretion.
Bioavailability of amino acids
The bioavailability of an amino acid is the proportion of ingested dietary AA that is absorbed in a chemical form suitable for it to be utilized for protein synthesis or metabolism( 6 , Reference Stein, Sève and Fuller 10 ). There is no direct method for measuring bioavailability and it is, therefore, generally estimated using measures of in-vivo digestibility or determined using slope-ratio assays, which give the bioavailability relative to a reference protein( Reference Stein, Sève and Fuller 10 ).
The utilization of an amino acid may be influenced by ingredient characteristics, genetics, physiological state and dietary factors( Reference Levesque, Moehn and Pencharz 11 ). Food processing can sometimes reduce the bioavailability of AA. One common example is Lys which has undergone Maillard reactions with reducing sugars or other aldehyde compounds during heat processing such as in heated skim milk powder( Reference Gilani and Sepehr 9 ): although it can be digested and partially absorbed by the body, it cannot be utilized for protein synthesis. Other changes associated with alkaline and/or heat processing include racemization of l-amino acids, and the formation of crosslinked peptide chains such as lysinoalanine, which result in a loss of Lys, Cys and Thr, together with reduced protein digestibility( Reference Sarwar 8 , Reference Gilani and Sepehr 9 ). Antinutritional factors can also cause a reduction in protein bioavailability. Some workers, therefore, suggest that digestibility as determined traditionally may not be a good approximation of bioavailability in products containing antinutritional factors present naturally or formed during processing or storage( Reference Gilani and Sepehr 9 ).
Overview of methods used for evaluating protein quality
A list of some of the principal methods used in evaluating protein quality is provided in Table 1, along with some considerations on their appropriateness and effectiveness. Methods frequently used include amino acid score (AAS), nitrogen balance (NB), in vivo protein digestibility (apparent, corrected or true), in vitro protein digestibility, protein efficiency ratio (PER), estimated PER, maximum PER, net protein ratio (or retention) (NPR), protein rating (PR), net protein utilization (NPU), biological value (BV) (apparent, true, relative) and the PDCAAS. Details on these different methodologies and their use have been extensively reported in the literature.
Table 1 List of some of the principal methods used in evaluating dietary protein quality

AAS is the ratio of the amino acid content in 1 g of a target protein to that of a reference protein or requirement. AA analysis, which is typically undertaken using ion exchange chromatography (IEC), gas chromatography (GC) or reverse phase high performance liquid chromatography (HPLC), is needed for calculating the amino acid content. A standard procedure for AA analysis was recommended by the 1989 Joint FAO/WHO Expert Consultation. This is because data can vary markedly depending on the method and conditions used. The WHO/FAO/UNU Expert Committee( 6 ) suggested that since in practice dietary proteins are likely to be limited only by Lys (most cereal proteins), the sulphur AA (legume proteins), Trp (some cereals such as maize) or Thr (some cereals), in calculating scores it is usually only necessary to use a pattern based on these four AA. In addition to variations in results if not properly calculated, major issues regarding the use of amino acid score include lack of information on AA bioavailability. Furthermore, processing can modify IAA which can influence digestibility. Additionally, the degree of amino acid absorption may depend on the length of peptide released after hydrolysis (limit peptides).
Nitrogen balance provides a measure of body nitrogen retention based on directly measuring daily nitrogen intake minus nitrogen excreted. Digestibility measurements estimate the extent to which proteins are hydrolysed by gastrointestinal enzymes into AA, and the extent to which these AA are absorbed, and provide a measure of the dietary protein intake which is made available to the organism after digestion and absorption. Protein efficiency ratio measures the ability of a protein to support the growth of young growing rats and is reported as the gain in weight per gram of protein consumed. As has been discussed extensively in the literature, rats have a higher need for sulphur containing IAA, thus, the PER method overestimates the requirements for humans and likely underestimates the quality of some proteins, especially plant proteins. NPR is similar to PER except that an additional factor (average weight loss of rats fed a non-protein diet) is taken into consideration. Protein rating is the product of the PER of a protein multiplied by the amount of protein in a reasonable daily intake. NPU provides a measure of overall protein utilization and reflects the proportion of ingested protein retained. Biological value, on the other hand, provides a measure of how well the absorbed amino acid profile matches that of the requirement. Challenges associated with BV include the following: results for the same food vary significantly depending on N intake; results for different foods may be similar at low N intake and very different at higher intake levels; proteins which are completely devoid of one IAA can still have a BV of up to 40 %; the method ignores the importance of factors which influence digestion of the protein and interaction of protein with other dietary factors before absorption.
The PDCAAS method is based on a determination of protein content, amino acid profile and protein digestibility. The limiting amino acid score (i.e., ratio of first limiting amino acid in 1 g of protein from the target food to that found in a reference protein or reference requirement) is multiplied by protein digestibility (true faecal digestibility) which gives a value for protein quality corrected for digestibility. The first limiting amino acid is the IAA present at the lowest concentration in a food. Depending on concentration, other IAA may be described as the second, third, fourth, (etc.), limiting amino acid. The recommended reference protein for infants under 1 year of age is that of human milk( 4 ). For all other age groups, the FAO/WHO 1991 report recommended the use of the amino acid scoring pattern for preschool children in the 2-5 year range. The 2007 WHO/FAO/UNU report, however, revised this recommendation and has suggested the use of the amino acid scoring pattern for preschool children in the 1-2 year range for preschool children, and the 3-10 yr pattern when judging protein quality for schoolchildren and adolescents.
Effects of bioactive compounds, preparation and processing conditions on protein quality
Protein quality of foods is affected by methods used in food preparation and processing and the presence of bioactive compounds. Foods are rarely consumed raw in today's fast paced society, and are often processed to increase convenience and safety, extend shelf life and improve taste. The quality of protein from both animal and plant sources is thus of most interest after preparation and processing. Plant-based foods are particularly of interest in this regard as they contain bioactives which in the unprocessed form can reduce digestibility and hence protein quality. Processing technologies could, however, help to transform raw grains into useful products which maximise their inherent nutritional value to ensure the nutrient security of populations, particularly, in developing countries( Reference Uppal and Bains 12 ). The impacts on protein quality of bioactive compounds and some of the principal techniques used in food preparation and processing are provided below.
Bioactive compounds
Antinutritional factors (ANF) can be naturally present in the food matrix in which a protein is consumed, or be formed during processing or storage. ANF can affect both the digestibility and bioavailability of protein and AA. Examples of naturally occurring ANF include phytates in cereals and oilseeds, polyphenols in pulses, gossypol in cottonseed protein products and glucosinolates in mustard and rapeseed proteins( Reference Sarwar 8 ). The limited digestibility of legume proteins has been ascribed to the presence of ANF such as protease, trypsin and amylase inhibitors (which can interact with digestive enzymes to form inactive complexes), tannins (that have a high affinity for Pro and His in proteins), lectins, phytic acid (which can form complexes with proteins, proteases and amylases, inhibiting proteolysis) and non-starch polysaccharides( Reference Nalle, Ravindran and Ravindran 13 , Reference Tavano, Da Silva and Démonte 14 ).
Processing methods such as soaking, cooking and fermentation have been shown to lead to better AA digestibility, most likely due to a reduction in ANF such as heat labile protease inhibitors( Reference Khattab, Arntfield and Nyachoti 15 ).
Germination
Sprouting of seeds which involves soaking seeds until germination, is used in many countries as part of food preparation. Germination significantly increases protein content and decreases starch level in bambara groundnut (Voandzeia subterranea)( Reference Abu, Ahmed and Abdalla Abdelsamad 16 ). Compared to other cooking methods (wet cooking and dry roasting), germination for 4-6 days significantly decreased phytic acid content and increased in vitro protein digestibility. The improvement of protein digestibility after germination was attributed to a reduction of polyphenols and phytic acid in the germinated seedling and an increase in soluble proteins due to the action of proteolytic enzymes. These enzymes were also effective in hydrolyzing protein-polyphenol complexes in the seed.
Kannan et al. ( Reference Kannan, Nielsen and Mason 17 ) also reported that germination increased true protein digestibility (TPD) of cooked black bean products, however, the increase was not accompanied by an increase in PDCAAS due to the limiting amino acid score. Highest TPD and PDCAAS values were obtained for cooked germinated beans combined with rice. Germination also had little effect on the amino acid profile of cowpeas( Reference Jirapa, Normah and Zamaliah 18 ). However, in vitro protein quality and starch digestibility improved after germination, resulting in a higher PDCAAS (still low) for a weaning food prepared from 24 h germinated cowpea flour (56 %) compared to the control cowpea flour weaning food (47 %).
In other studies, germination promoted an increase of 21 % in the protein digestibility of sorghum proteins and an increase in protein extractability( Reference Correia, Nunes and Barros 19 ). Protein content of mungbean, chickpea and cowpea also increased by 9-11, 11-16 and 8-11 %, respectively after germination, and in vitro protein digestibility similarly increased by 15-25, 6-17 and 6-17 %, respectively with higher improvements observed with longer times of germination( Reference Uppal and Bains 12 ).
Higher increases in in vitro digestibility of Indian bean (Dolichos lablab. var. lignosus) seeds in the early stage of germination were also reported( Reference Ramakrishna, Jhansi Rani and Ramakrishna Rao 20 ). PER values increased in rats fed with the germinated bean, reaching comparable values with a control group maintained on a casein diet after 32 h of germination. Diets with germinated bean protein also showed marked increases in both true and apparent nitrogen digestibility, although the values were less than that observed for casein fed rats.
In addition to protein, germination also caused significant increases in thiamine, in vitro iron and calcium bioavailability and in vitro protein digestibility of green gram (Phaseolus aureus), cowpea (Vigna catjang), lentil (Lens culinaris) and chickpea (Cicer arietinum)( Reference Ghavidel and Prakash 21 ). Dehulling the germinated legumes yielded further increases in nutritive value. Phytic acid and tannin were reduced by 18-21 % and 20-38 %, respectively, on germination and further reduction was observed on dehulling. The low levels of phytic acid and tannin detectable in the cotyledons after dehulling, suggest that most of the phytic acid and tannin were present in the seed coat. Negative correlations were, however, reported between antinutritional factors and nutrient bioavailability and digestibility.
Osman( Reference Osman 22 ), however, noted that germination significantly increased tannin content in another legume (Dolichos lablab bean [Lablab purpuresus (L) sweet]) compared with other traditional methods of food preparation, although germination was more effective in improving protein digestibility than soaking and cooking. Similarly, germination of kidney bean (Phaseolus vulgaris L.) was less effective in reducing trypsin inhibitors, saponins and phytohaemagglutinins than cooking/autoclaving( Reference Shimelis and Rakshit 23 ). Germination, however, reduced stachyose, raffinose, phytic acid and tannins. The combination of germination followed by autoclaving resulted in a 100 % reduction of phytic acid and tannin and a 9-18 % increase in in vitro protein digestibility.
Wet or moist thermal treatment
In plant proteins heat processing, especially moist heat, may sometimes improve protein digestibility by destroying protease inhibitors and denaturing proteins, which can open up their structure allowing gastrointestinal enzymes greater access for hydrolysis. Soaking, boiling, microwave cooking and autoclaving increased total IAA content determined in the seeds of cowpea, pea and kidney bean( Reference Khattab, Arntfield and Nyachoti 15 ). The magnitude of the effect was in the following order: autoclaving>boiling >microwave cooking>soaking. The determined level of sulphur AA also slightly increased in all samples after microwave cooking and autoclaving, and autoclaving was found to be most effective for improving protein efficiency ratio and amino acid score (based on assumption of Cys+Met as being first limiting), followed by micronization, microwave cooking and fermentation. In vitro protein digestibility also significantly improved after soaking, boiling, microwave cooking, pressure cooking (autoclaving) and fermentation (Table 2). The PDCAAS decreased in the order microwave cooking>autoclaving>boiling>soaking, with some exceptions. Very large differences in PDCAAS were observed depending on processing: for example, the PDCAAS of raw Canadian kidney beans was half the value of the PDCAAS from microwave cooking.
Table 2 Effects of processing conditions on protein quality (adapted from Khattab et al. ( Reference Khattab, Arntfield and Nyachoti 15 ))

Saleh and El-Adawy( Reference Saleh and El-Adawy 24 ) similarly reported significant decreases in antinutritional factors (trypsin inhibitor, haemagglutinin activity, tannins, saponins and phytic acid) of chickpea (Cicer arietinum L.) after cooking (i.e., using microwave cooking and other traditional cooking methods). In vitro protein digestibility (IVPD) and protein efficiency ratio were improved by all cooking treatments from 84 % (raw) to approximately 90 % for IVPD and 2·3 (raw) to approximately 2·5 for PER. Chemical score and the amino acid determined to be first limiting for chickpeas subjected to the various cooking treatments, however, varied considerably, depending on the type of treatment.
In other studies, saponins, trypsin inhibitors and phytohaemagglutinins, diminished dramatically to undetectable amounts when kidney beans (Phaseolus vulgaris L.) were cooked or autoclaved( Reference Shimelis and Rakshit 23 ). Similarly, cooking of pre-soaked seed was found to be the most effective method for reducing trypsin inhibitor activity in Dolichos lablab bean( Reference Osman 22 ) and a significant increase in IVPD was also found when raw sprouts of mungbean, chickpea and cowpea were subjected to pressure cooking and microwaving( Reference Uppal and Bains 12 ).
Heat treatment can sometimes, however, be detrimental to protein quality. As an example, digestibility and extractability of sorghum proteins decreases, especially, on wet cooking( Reference Correia, Nunes and Barros 19 , Reference Duodu, Taylor and Belton 25 ). This occurs due to binding of the tannins to proteins in tannin-containing sorghum cultivars. Tannins have antioxidant properties and are bacteriostatic and/or bactericidal for many bacteria species( Reference Scalbert 26 ). Although the positive benefits of tannins are increasingly being recognised, their presence in high amounts in plant based foods unfortunately has a detrimental effect on protein quality. The reduced digestibility of cooked sorghum products has also been attributed to disulphide crosslinks occurring between γ- and β-kafirin proteins at the protein body periphery, which may impede digestion of the centrally located major storage protein, α-kafirin( Reference Duodu, Taylor and Belton 25 ).
Milk sterilization (at 110-120°C, 20-30 min) also causes a decline in protein quality, due to a decrease in Lys, Met and Cys( Reference Boschi, Traisci and Siervo 27 ). Maillard reactions involving Lys, and destruction of the sulphonic group in the case of Met and Cys due to sterilization, may be the cause. PDCAAS for sterilized semi-skimmed milk was 34 % whereas it was much higher for pasteurized semi-skimmed milk (76 %). In general, no appreciable Maillard reaction is expected to occur during pasteurization, and only very small losses of Lys have been reported ranging from 0-5 % on ultra high temperature (UHT) treatment( Reference Andersson and Öste 28 ).
In another study where hot water (80°C) reconstituted powdered infant formulas were sterilized by autoclaving for 5 min at 105°C, a 20 % reduction in total protein was found after autoclaving compared with the conventional preparation, where samples were reconstituted with warm water (37°C) in glass bottles but not autoclaved( Reference Yeung, Lee and Lin 29 ). Concentrations of total free AA and in particular some specific individual amino acids, Val ( − 72 %), Gln ( − 60 %) and Lys ( − 40 %), also decreased in the autoclaved formulas. Higher concentrations of ammonia found after autoclaving suggested degradation of protein and AA.
Dry heating
Dry heat treatment of protein flours includes processing treatments such as dry roasting and micronization (a thermal treatment based on infrared heating). Despite the heat processing applied, dry roasting and micronization reduced the IVPD of cowpea, pea and kidney bean by 5·72–7·96 and 2·75–3·72 %, respectively, when compared with raw legumes( Reference Khattab, Arntfield and Nyachoti 15 ) (Table 2). IAAs and PDCAAS, however, increased after roasting and micronization for the majority of seeds studied. The reduction in protein digestibility was attributed to non-enzymatic browning (Maillard reaction) between the reducing sugars from starch hydrolysis and the proteins, as well as the thermal cross-linking that occurred during heating( Reference Khattab, Arntfield and Nyachoti 15 ). For sorghum proteins a decrease of 4 % in protein digestibility was observed after dry heating and protein extractability was not affected( Reference Correia, Nunes and Barros 19 ).
Smoking and broiling
Essential AA in rainbow trout were reported to be generally much higher in raw samples than after smoking and broiling( Reference El and Kavas 30 ). Lys was particularly affected and in overheated fish, was drastically reduced compared to untreated fish. Compared with raw rainbow trout, broiling reduced the digestibility of protein (i.e., % decrease) by 1·63-3·9 % and smoking by 4·2-4·5 %. PDCAAS of raw trout was reduced by 6 % after smoking and by 3 % after broiling. The decrease in protein quality was attributed to an increase in SH (sulphydryl) groups and S-S bonds, as well as complex chemical (cross-linking) reactions such as protein-protein interactions or protein-fat interactions when food was broiled at high temperatures. Smoking conditions (time, temperature, compounds of wood smoke) are all factors that can negatively influence protein digestibility.
Bender( Reference Bender 31 ) showed that at the rather low temperature needed to cook meat there is little loss of available Lys and no loss of Met and Cys. No change in protein quality was found after roasting meat in an open pan at 163°C when the internal temperature did not rise above 80°C; or when the meat was browned in an oven for 30 min then sterilised in a can( Reference Bender 31 ). When meat is roasted the outer part reaches a high temperature and turns brown (Maillard reaction) which produces the desired roast flavour but since the roasted part is only a small fraction of the total piece of meat and, especially, when the internal temperature does not exceed about 80°C, there is no measurable change in the quality of the protein as a whole( Reference Bender 31 ).
In another study( Reference Okonkwo, Obanu and Ledward 32 ), intermediate moisture smoked beef was prepared by cook-soak/equilibration, where samples were either smoked for 18 h (heavy smoking) or for 4 h (light smoking) at 50°C. Smoking caused a marked decrease in SDS-soluble protein and slightly decreased the available Lys and percent conversion of the haemoproteins to the cured nitrose forms. Smoking also caused increased darkening and hardness of the samples and a slight loss of some of the protein components.
Evans et al. ( Reference Evans, Carruthers and Witty 33 ) compared the protein quality of meats after standard cooking procedures by assessing the effects on relative nutritive value (RNV) and amino acid composition. Boiled tissue meats and processed meat showed higher RNV and levels of essential AAs than fried or microwave cooked or uncooked samples. However, in organ meats, cooking did not change total protein content or total essential amino acid contents relative to uncooked organ meats.
Spray-drying
Spray-drying is a downstream unit operation frequently used in the food industry to extend the shelf life of foods. Liquid foods are pumped through the nozzle of the spray dryer and brought into intimate contact with a counter current flow of hot air which causes flash vaporization of moisture leaving a shelf stable powdered particulate material. Spray drying is used for drying caseinates, whey protein, soya proteins and a variety of other products and the temperatures and times used can vary extensively for different food products. Concerns about the impact of spray-drying on protein quality include the occurrence of Maillard reactions, degradation of AA and possible conversion of L- to D- AA amongst others.
In one study where the nutritional protein quality of lactose-hydrolysed milk was studied with N balance experiments on growing rats, the authors found that spray-drying under conditions usually used for ordinary milk gave a considerable reduction in protein quality, caused mainly or entirely by loss of biologically available Lys( Reference Burvall, Asp and Dahlqvist 34 ).
A higher spray drying temperature was also found to significantly decrease protein quality and the contents of all AA in a spray-dried protein hydrolysate from black tilapia fish( Reference Abdul-Hamid, Bakar and Bee 35 ). In vitro protein digestibility decreased from 92 % (150°C/76°C inlet/outlet temperature) to 88·4 % (180°C/90°C inlet/outlet temperature) and PDCAAS % decreased from 82 to 34, respectively with increasing processing temperature. Additionally, predicted protein efficiency ratios of the dried hydrolysates decreased from 2·97 to 2·53.
Extrusion
Extrusion is a high temperature high shear process used in food texturization which has grown in popularity in recent times. It is used extensively in the processing of snacks, cereals, meat and a variety of other products. The high temperatures and shear pressures used during extrusion can denature or degrade proteins and AA and impact protein quality either positively or negatively.
Extrusion of pea seeds (Pisum sativum L. var. laguna) increased protein recovery slightly and decreased trypsin inhibitory activity (TIA) to negligible levels( Reference Frias, Giacomino and Peñas 36 ). Val, Phe and Lys contents decreased significantly (10–22 %) when extrusion was carried out at 129°C, whereas Trp decreased only when higher temperatures were used (142°C). Changes were also observed in the concentrations of dietary dispensable AA, with Pro undergoing the greatest reductions (28-38 %), followed by Gly (10-15 %). Interestingly, biological indices for protein quality (NPU, TD, BV, NPR), remained unchanged after extrusion, but PDCAAS decreased from 66 (raw flour) to 55, 61, 59 % for samples extruded at 129, 135 and 142°C, respectively.
Digestibility of hard-to-cook flour of cowpea was also improved by 56 % after extrusion( Reference Batista, Prudêncio and Fernandes 37 ). Furthermore, extrusion at 150°C significantly decreased antinutrients such as phytic acid (33·2 %), lectin (100 %), α-amylase (100 %), and trypsin inhibitors (38·2 %). In vitro protein digestibility of common bean (Phaseolus vulgaris L.) was also improved by 72·3–84·5 % after extrusion( Reference Batista, Prudêncio and Fernandes 38 ).
Also, extrusion did not alter IVPD of soyabean and corn; however, it reduced the amount of trypsin inhibitors when a combination of the grains was extruded at 120°C( Reference Bertipaglia, DeMelo and Sugohara 39 ). Proximate composition of extruded products from corn (Zea mays) and lima bean (Phaseolus lunatus) flour blends showed increased protein and ash contents whereas fat levels decreased. The IVPD of the extrudates increased to 82 % compared to the raw flours (77 %)( Reference Pérez-Navarrete, Betancur-Ancona and Casotto 40 ). Other reports in the literature suggest that extrusion conditions targeting aflatoxin reduction in peanut does not adversely affect protein nutritional quality.
In contrast, Silva et al. ( Reference Silva, Cruz and Arêas 41 ) found that extrusion of bovine rumen protein containing about 96 % protein significantly reduced true protein digestibility from 97·7 % to 93·1 %. The limiting amino acid also changed after extrusion but scores remained similar (i.e., 1·28 (Leu) for raw bovine rumen and 1·25 (Met + Cys) for the extruded sample). Un-truncated protein digestibility-corrected amino acid scores decreased from 125 to 116 % after extrusion. In this particular example, for both raw and extruded proteins, PDCAAS values were, however, excellent (100 %) and animal growth profiles using raw and extruded rumen were also found to be comparable.
Irradiation
Food irradiation is a processing technique in which foods are subjected to ionising radiation to destroy insects and other pathogens of microbial origin and which affects food quality as well as safety. Several countries permit the use of food irradiation for safety reasons. Foods can be treated using low dose (>2 kGy), medium dose (2-10 kGy) and high dose (>10 kGy) radiation. Low dose irradiation is used to delay sprouting of vegetables and aging of fruits. Medium dose radiation helps to reduce levels of pathogenic organisms whereas high doses are used for food sterilisation. Food irradiation is not permitted for use to increase the nutritional value of foods; nevertheless some authors have evaluated its impact on protein quality. Bhat and Sridhar( Reference Bhat and Sridhar 42 ) studied the impact of electron beam irradiation on the nutritional and anti-nutritional properties of lotus seeds. Their results showed a higher concentration of IAAs (Thr, Val, Leu, Tyr + Phe, and Ly) after irradiation. PDCAAS, however, significantly decreased after irradiation due to a decrease in IVPD which went from 43 % for the untreated sample to 24 %, after application of 30 kGy irradiation. The decrease in IVPD was dose dependent: (irradiation dose kGy: IVPD % 0:43, 2·5:41, 5:40, 7·5:40, 10:40, 15:41 and 30:24) and was found to be statistically significant only at 30 kGy. Radiation levels ranging from 30 kGy to 75 kGy have been used for example for microbial disinfection of spices and seasonings, sterilisation of frozen packaged meats for astronauts, and meals for immuno compromised hospital patients.
Fermentation
Food fermentation has been used in many cultures since ancient times to preserve food and improve taste. The fermentation process involves the use of a variety of micro-organisms such as bacteria, moulds and yeasts which may be naturally present in or on the food or expressly added to induce fermentation. Fermentation provides a technological alternative for improving the nutritional value of a great variety of legumes and cereals while maintaining acceptable sensory properties( Reference Cuevas-Rodríguez, Verdugo-Montoya and Angulo-Bejarano 43 ). The micro-organisms used in fermentation synthesise enzymes which hydrolyse food constituents and contribute to the development of products with desirable organoleptic properties. Furthermore, the hydrolysis could contribute to the decrease or elimination of anti-nutritional factors which could help in improving nutritional quality of the food.
Angulo-Bejarano et al. ( Reference Angulo-Bejarano, Verdugo-Montoya and Cuevas-Rodríguez 44 ) reported an improved protein digestibility of chickpea flour after solid-state fermentation (SSF). In this study, chickpea seeds were soaked, seed coats removed, and cotyledons were cooked at 90°C for 30 min and inoculated with a suspension of R. oligosporus and fermented at 34·9°C for 51·3 h followed by drying at 52°C for 12 h and milling. Proteins from unfermented and fermented flours had IVPD of 72 % and 83 %, respectively. True protein digestibility (in vivo) increased form 84 % to 89 % and PER, NPR and PDCAAS improved from 1·6 to 2·3, 2·7 to 3 and from 73 to 92 %, respectively, as a consequence of the cooking/fermentation process. The improvement of PER during fermentation was attributed to better availability of AA and greater digestibility of the proteins in the substrates. Total sulphur AA (Met + Cys) was the first limiting IAA in proteins from untreated chickpea with an IAA score of 0·87. In the fermented flour, Trp was the first limiting IAA with an IAA score of 0·93. The essential AA content of untreated chickpea was improved by the SSF process including levels of Ile, total sulphur AA (Met + Cys), total aromatic AA (Phe + Tyr), and Thr. The control sample for this study was, however, raw uncooked flour. To quantify the effect of fermentation it would have been useful if the authors had provided the protein quality results of the flour, cooked without the fermentation step.
In another similar study( Reference Cuevas-Rodríguez, Verdugo-Montoya and Angulo-Bejarano 43 ), SSF increased the content of IAA in maize from 41 to 49 g IAA/100 g protein. His, Ile, Leu, Lys and Trp increased by 0·8, 0·5, 1·5, 1·5 and 0·12 g/100 g protein, respectively. Total sulphur AA (Met & Cys) and total aromatic AA (Tyr & Phe) increased by 0·6 and 3·5 g/100 g protein, respectively. Some AA levels, notably Val, however decreased from 6·1 to 4·3 g/100 g protein. First and second limiting IAAs in the untreated flour were Lys (0·72) and Trp (0·73), but this changed after fermentation to Trp (0·84) and Lys (0·98), respectively. Overall, fermentation increased protein quality indicators as follows: true protein digestibility from 76·6 to 86·8 %, protein efficiency ratio (PER) from 1·8 to 2·1 and PDCAAS from 55 to 83 %.
Nicolau et al. ( Reference Nicolau, Georgescu and Segal 45 ) found the protein content in boiled rice doubled after solid state fermentation using a strain of Saccharomycopsis fibuligera, an amylase producing yeast. The increase was attributed to the yeast biomass which contributed protein rich in Lys (an amino acid limiting in rice), Met and Trp. The fermented rice was enriched in B group vitamins (B1, B2, and B6) synthesized by the yeast and phosphorus bioavailability also increased as result of fermentation. Fermentation also promoted an increase of 39·6 % in the protein digestibility of sorghum proteins and an increase in protein extractability( Reference Correia, Nunes and Barros 19 ).
A tempeh-type fermented product was susscesfully prepared from fresh and hardened common beans( Reference Paredes-López and Harry 46 ) using Rhizopus oligosporus. Soluble solids, total and soluble proteins, soluble carbohydrates and pH of both bean samples increased after fermentation whereas fat and fibre content decreased. Trypsin inhibitor units (TIU/g d.b) decreased from 120,000 – 130,000 in raw beans to 61,090 – 65,000 after soaking/dehulling/cooking (SDC) to 250 – 900 after fermentation. Phytic acid levels did not change much by the SDC treatment but significantly decreased from 2·1 to 1·4 g/100 g d.b after fermentation. Cooking also reduced the lectin level significantly to almost undetectable levels and as a result this value did not change much after fermentation. The results suggest that the Rhyzopus oligosporus used for fermentation was capable of hydrolyzing the trypsin inhibitor and phytic acid of the substrate which may explain the improvements in protein quality in the previous studies reported above.
More recently, Fagbemi et al. ( Reference Fagbemi, Oshodi and Ipinmoroti 47 ) also found fermentation to be the most effective processing method to reduce phytic acid and trypsin inhibitor activity in mature seeds of breadnut, cashew nut and fluted pumpkin, whereas boiling was most effective in reducing tannin content. Boiling, fermentation, germination and roasting reduced TIA of the seed flours by 20·4, 88·7, 0·9 and 26·8 % (breadnut); 57·1, 67·1, 34·5, and 58·7 % (cashew nut); 100, 100, 94 and 100 % (pumpkin), respectively. Boiled samples had the highest IVPD (most digestible) followed by the fermented samples.
Interestingly, not all authors have observed increases in nutritional quality after fermentation. Kannan et al. ( Reference Kannan, Nielsen and Mason 17 ) found no statistically significant increase in either TPD or PDCAAS values upon fermentation of black bean products.
In the dairy sector, proteins in yogurt, acidophilus milk, and bifidus milk have been found to be more digestible than those in unfermented milk( Reference Lee, Friend and Shahani 48 ). The enhanced digestibility is attributed to protein denaturation and hydrolysis during fermentation, which results in the formation of smaller, more digestible curds. Pre-hydrolysis of milk proteins, as indicated by increased levels of free AA, especially Pro and Gly, occurs during the manufacture of yogurt( Reference Adolfsson, Meydani and Russell 49 ). Cultured yogurt has a significantly higher protein quality than the mix from which it is made as evidenced by in vivo digestibility, net protein ratio and computed protein efficiency ratio( Reference Lee, Friend and Shahani 48 ). The activity of proteolytic enzymes and peptidases is apparently preserved throughout the shelf life of yogurt: the concentration of free amino groups increases up to twofold during the first 24 h and then doubles again during the next 21 d of storage at 7°C( Reference Adolfsson, Meydani and Russell 49 ). Some bacterial cultures have greater proteolytic activity during milk fermentation and storage than others (as indicated by elevated concentrations of peptides and free AA after milk fermentation)( Reference Adolfsson, Meydani and Russell 49 ). Both heating and fermentation reportedly contribute to the high protein quality of yogurt.
Overall, when a summary of the evidence is taken into consideration, fermentation may be a useful processing technique to reduce anti-nutritional factors in plant based proteins which could contribute to improving protein digestibility as well as protein quality. For animal protein as well, fermentation may contribute to further improvements in quality.
Protein quality evaluation methods and recommended intakes currently used in different countries
Various terminologies are used in the literature to describe nutritional requirements. Table 3 provides a description of some of the most frequently used ones. To promote adequate nutrition, countries around the world provide recommendations for intake of different nutrients including protein. As proteins play a critical role in health, methods to assess their quality, efficient methods of processing to enhance their nutritive value and safe levels of intake need to be established. Some country specific protein recommended intakes and quality evaluation methods are provided in Table 4. Average and safe levels of protein intake recommended by the WHO/FAO/UNU( 6 ) are shown in Table 5 and recommendations for essential AA in Table 6.
Table 3 Different terminologies used in the literature to describe nutritional requirements

Table 4 Country specific recommended protein intakes and quality evaluation methods

Table 5 WHO/FAO/UNU (2007)6 recommended average and safe levels of protein intake
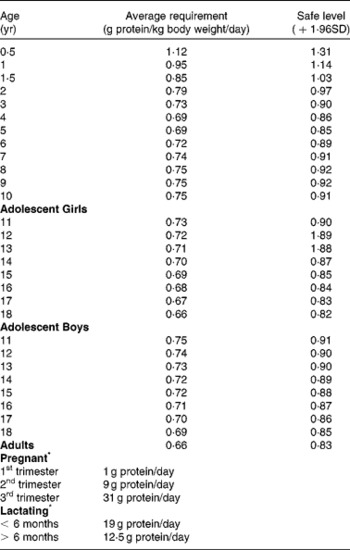
Table 6 Suggested patterns of human dietary indispensible amino acid requirements

It is important to note that the recommended dietary intake (RDI) is set at 1·96 times the standard deviations (SD) above the estimated average requirement (i.e., when requirement for the nutrient is symmetrically distributed) in order to meet the nutrient requirements of nearly all (97–98 %) healthy individuals in a particular life stage and gender group. The National Health and Medical Research Council (NHMRC)( 50 , 51 ) in Australia defines RDI as "the levels of intake of essential nutrients considered,… on the basis of available scientific knowledge, to be adequate to meet the known nutritional needs of practically all healthy people … they incorporate generous factors to accommodate variations in absorption and metabolism. They therefore apply to group needs. RDIs exceed the actual nutrient requirements of practically all healthy persons and are not synonymous with requirements".
Interpretation of the RDI especially in relation to evaluating protein quality and determining appropriate amounts for labeling purposes can sometimes be challenging for consumers and industry. The Codex Alimentarius and some countries (e.g., USA) therefore provide specific guidelines( 52 , 53 ). Furthermore, as processing, matrix and the presence of bioactives can influence protein extraction, AA recovery, AAS, the first limiting amino acid and AA digestibility, it is important that these be taken into consideration in the evaluation of protein quality and in establishing labelling guidelines.
Protein quality of some key food groups
Tables 7–9 provide a list of protein quality indices for various food groups and food blends. Foods are frequently consumed in complement with other foods which raises questions as to the significance of the PDCAAS values of single protein sources. Practically all animal sources of protein have PDCAAS values equal to or above 1 (or 100 %). The excess IAA provided by these foods could be useful in complementing negative IAA balances in other foods with lower protein quality.
Table 7 Protein digestibility-corrected amino acid score (PDCAAS) for cereals, meat proteins, vegetables and tree nutsa

Table 8 Protein digestibility-corrected amino acid score (PDCAAS) for some legumesv
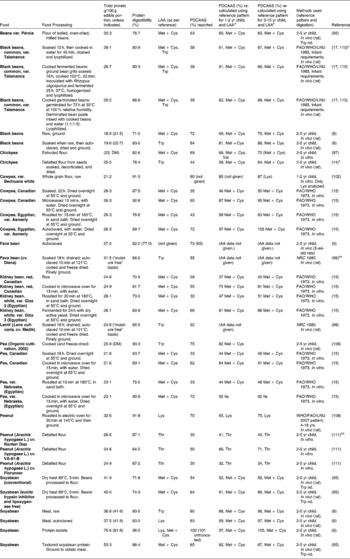
Table 9 Protein digestibility-corrected amino acid score (PDCAAS) of some composite productsa
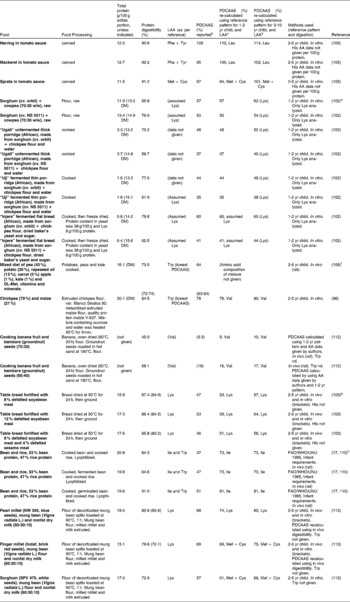
Although some plant sources of protein, such as soya protein isolate and soyabean have PDCAAS values close to 100 %, many other sources of plant protein have PDCAAS values that are much lower. Large variations in PDCAAS are evident even within food groups: for example, where pearl millet and sorghum have PDCAAS values as low as 20 %, one variety of quinoa was reported to have a PDCAAS as high as 100 % (Table 7). Similarly, among tree nuts, almonds (Prunus dulcis L.) have a PDCAAS of < 25 % while Baru almonds (Dipteryx alata Vog.) and tropical almonds (Terminalia catappa) have a value that is almost three times higher. The advantage of combining food proteins is evident from Table 9: by mixing sorghum and finger millet with 30 % mung bean flour and 10 % nonfat milk, the PDCAAS values are increased 2-3 fold with respect to those for the grains on their own.
Concerns have been raised by some about the adequacy of plant sources of protein to provide dietary protein requirements. The American Dietetic Association( 54 ) has indicated that an assortment of plant foods eaten over the course of a day can provide all dietary essential AA and ensure adequate nitrogen retention and use in healthy adults, thus complementary proteins do not need to be consumed at the same meal. The ADA further clarifies that although some vegan women have protein intakes that are marginal, typical protein intakes of lacto-ovo-vegetarians and of vegans when well planned appear to meet and exceed requirements and can also provide adequately for the protein needs of athletes.
Key issues still requiring consideration in protein quality evaluation
There still remains a myriad of issues requiring consideration in relation to the evaluation of protein quality. A few of these issues are provided below.
Amino acid scoring patterns
The amino acid scoring patterns in use at the present time determine the effectiveness with which absorbed dietary nitrogen can meet the indispensable amino acid requirement at the safe level of protein intake( 5 , 6 ). The safe level of intake as indicated above is set at 1·96 times the SD above the estimated average requirement. Further discussions and considerations are required as to whether the scoring patterns should be based on amino acid requirement values divided by the mean protein requirement or the safe level of protein intake. Further reflection is also required on the AA requirements of infants vs. adults (i.e., growth vs. maintenance). Infants and children have need for positive nitrogen balance to sustain growth whereas adults need nitrogen equilibrium to maintain health. Questions have also been raised about specific requirements for each of the sulphur containing IAA. Furthermore, from a practical standpoint, food regulators need to consider that it may be beneficial for industry if PDCAAS could be calculated using only one reference pattern (i.e., 1-2 yr or 3-10 yr pattern).
PDCAAS truncation
For higher quality proteins having PDCAAS values greater than 1 or 100 %, the current recommendation is to truncate the value to the maximum value of 1. As a mixture of proteins may be consumed in the diet over any given period of time in the course of a day, the extra IAA provided by higher quality proteins could complement proteins lacking IAA. The description of this benefit is lost with truncation. There are also questions in regards to which value to truncate, i.e., the PDCAAS or the amino acid score prior to multiplying by the digestibility factor. The FAO/WHO 1991 Expert Consultation recommended the former whereas the WHO/FAO/UNU 2007 Expert Consultation argued, that on the basis that digestibility is first limiting, the PDCAAS value should be calculated from a truncated amino acid score value. From a biological and practical perspective, AA released after digestion of a given amount of protein will be based on the total amount of AA initially present in the protein and not on a truncated amount, thus the FAO/WHO 1991 recommendation to truncate the PDCAAS score rather than the AA score seems valid. Further discussions are evidently required on this issue.
Calculation of the amino acid score for a dietary protein mixture
In calculating the amino acid score for a food protein mixture, where the digestibility of individual constituents varies, the FAO/WHO 1991 Expert Committee recommended calculating the PDCAAS using a weighted average protein digestibility and AA score calculated from the weighted amino acid content per gram of dietary protein. The WHO/FAO/UNU 2007 report recommended that the composition and amino acid score of the absorbed available AA in a mixture will reflect the relative digestibility of the individual food protein constituents, thus the amino acid score for food mixtures should be calculated from the weighted average digestible amino acid content. This is valid. However, a concern with the example provided in the WHO/FAO/UNU 2007 report is that digestibility could be construed to have been used "twice" not "once" in calculating the PDCAAS (first in calculating available protein, and then secondly to calculate the PDCAAS). A simpler recommendation would be to base the digestible amino acid value on the original amount of protein present in the diet rather than on the digestible protein which would make the second use of the digestibility function unnecessary.
Use of faecal vs. ileal digestibility
The FAO/WHO 1991 Expert Committee recommended the standardized rat faecal-balance method as the most suitable practical method for predicting protein digestibility. As pointed out by the WHO/FAO/UNU 2007 report, although faecal digestibility is probably the most appropriate measure of overall nitrogen digestibility, it is unlikely to be a true measure of amino acid digestibility. Digestibility measurements at the ileal level may provide a better measure of amino acid digestibility, however this may pose significant challenges for many researchers.
Use of in vivo vs. in vitro techniques in calculating protein digestibility
Animal studies to determine true protein digestibility can be expensive, thus, cheaper in vitro methods that accurately estimate true protein digestibility are needed. Hsu et al. ( Reference Hsu, Vavak and Satterlee 55 ) and Satterlee et al. ( Reference Satterlee, Marshall and Tennyson 56 ) developed a multienzyme in vitro system consisting of trypsin, chymotrypsin and peptidase. The pH of a protein suspension immediately after 10 min digestion with the multienzyme solution at 37°C( Reference Hsu, Vavak and Satterlee 55 ), or after an additional l0 min incubation with microbial protease at 55°C( Reference Satterlee, Marshall and Tennyson 56 ) was highly correlated with the in vivo apparent faecal digestibility of rats (0·90 with a standard error of estimate of 2·23 for the first study). Pedersen and Eggum( Reference Pedersen and Eggum 57 ) developed a pH-stat assay in which initial rate of alkali consumption is used to calculate a rate of hydrolysis of peptide bonds. McDonough et al. ( Reference McDonough, Sarwar and Steinke 58 ) also standardized a pH-stat method for in vitro digestibility.
Various workers have reported good correlations between some of the in vitro methods proposed and in vivo digestibility( Reference El and Kavas 30 , Reference Bodwell, Satterlee and Hackler 59 – Reference Marletta, Carbonara and Carnovale 62 ). Some legumes, however, appear to have higher in vitro values compared to the in vivo values. Carias et al. ( Reference Carias, Cioccia and Hevia 63 ) used three in vitro methods (pH drop, pH stat and pepsin digestibility) and two in vivo methods (true and apparent faecal digestibility in rats) to compare the protein digestibility of casein, soya protein isolate, fish meal, black beans, corn meal and wheat flour. All methods were in agreement for highly digestible proteins but less so for proteins with digestibilities below 85 %. They recommended that for non-conventional proteins or for known proteins subjected to processing, protein digestibility should be measured in vivo. Further studies are needed to ascertain the conditions under which in vitro digestibility methods can estimate apparent and/or true protein digestibility. Furthermore, the FAO/WHO 1981 recommended that amino acid scores be adjusted for "true" protein digestibility, thus the relevance of using "apparent" protein digestibility (or equivalents) should be considered. Similarly, as some studies have shown that apparent digestibility varies with the level of protein intake, some consideration should be given as to how in vitro digestibility methods estimating apparent digestibility are affected by protein concentration. In addition, some emerging research suggests that the rate of protein digestion may also be of importance in protein quality( Reference Millward, Layman and Tomé 7 ).
Protein vs. amino acid digestibility
Digestibility and bioavailability of AA influence protein quality. There are concerns that protein digestibility measurements may not provide accurate estimation of the digestibility of specific IAA. Thus, research is needed to determine to what extent protein digestibility as a whole (in vivo or in vitro) reflects the digestibility of specific IAA and how this may be affected by processing, matrix effects and other biotic and abiotic factors. Further research is also needed on the impact of processing on the bioavailability of specific essential AA.
Impact of processing and anti-nutritional components on protein quality
Several workers have reported that the presence of anti-nutritional components in some protein sources can influence their digestibility. Gilani and Sepehr( Reference Gilani and Sepehr 9 ) concluded from protein digestibility studies using young and old rats that the use of young rats may overestimate protein quality for the elderly for proteins containing antinutritional factors. The magnitude of the effect varied by protein type as well by processing treatment when the same protein source was subjected to different treatments, which suggests both protein and processing effects. Interestingly, research is also emerging on potential health benefits of some of these "bioactive" compounds which raises questions about whether their consumption to some extent may be beneficial.
Formation of tannin-protein complexes, as an example, have shown antioxidant properties acting as potent radical cation scavengers which could make them radical sinks in the gastrointestinal tract( Reference Riedl and Hagerman 64 ). Tannins also have antimicrobial properties. Various mechanisms for the tannin antimicrobial activity have been suggested, including inhibition of extracellular microbial enzymes, deprivation of substrates required for microbial growth, or direct action on microbial metabolism through the inhibition of oxidative phosphorylation( Reference Scalbert 26 ).
In vivo and in vitro experiments have demonstrated marked anticancer (preventive as well as therapeutic) effects of inositol hexaphosphate (IP6, phytic acid)( Reference Shamsuddin 65 ). IP6 reduced cell proliferation and increased differentiation of malignant cells resulting sometimes in reversion to the normal phenotype. Phytic acid may also be beneficial by reducing the bioavailability of toxic heavy metals such as cadmium and lead, and reducing excessive oxidation activity of iron and copper through chelation( Reference Campos-Vega, Loarca-Pina and Oomah 66 ). Kunitz trypsin inhibitors and Bowman-Birk inhibitors isolated from legumes have been shown to function as therapeutic agents against digestive system cancer and in ulceratitis prevention, and may contain anti-inflammatory activity and anti-viral activity( Reference Ribeiro, Cunha and Fook 67 ).
As it is still unknown at what concentrations these intakes may be beneficial and the specific mechanisms at play, further research specifically regarding how the presence of these so-called antinutritional compounds are affected by processing and the impact of both factors on protein quality will be useful.
Conclusion
Significant progress has been made in the last half century in defining protein quality and establishing appropriate levels of intake to support growth and maintain health. As results from new research emerge, recommendations may need to be updated or revised to maintain relevance. Changes in lifestyle, energy expenditure and new challenges in populations with high disease burdens require constant surveillance. To keep guidelines and legislations relevant, new scientific data will be required to support policy and inform expert recommendations at the global level. Some studies, for example, suggest that calorie intake and frequency of protein consumption influences nitrogen retention and should be considered in protein quality evaluation. Several studies have also shown that over and above that of basic nutrition, additional health benefits may be provided by specific amino acids and bioactive peptides( Reference Millward, Layman and Tomé 7 , Reference Kong and Xiong 68 – Reference Katsanos, Kobayashi and Sheffield-Moore 81 ). On the other hand, disease risks associated with over consumption of protein and potential differences in effects of different proteins in stimulating disease is poorly understood and requires further research( Reference Millward 82 ). Additionally, an important issue that will require consideration is whether the recommended protein intakes for public health purposes are based on assumptions of a particular protein quality of the diet. This is of interest as dietary protein quality can vary depending on social, economic and other geographic factors. Further discussions are also needed on the practical interpretation and use of the term 'safe' or 'upper level' to describe recommended protein intakes. For example, the 2007 WHO/FAO/UNU Report( 6 ) notes that "the term 'safe intake' also includes the concept that there is no risk to individuals from excess protein intakes up to levels considerably above the safe intake". As we move towards the year 2050 and beyond, particular challenges around the paradox of under-nutrition/malnutrition and over-nutrition, especially as related to protein intake and consequent disease burdens, will also need to be given greater attention.
Which Of The Following Cooking Methods Would You Use To Increase The Digestibility Of Protein?
Source: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/protein-quality-evaluation-twenty-years-after-the-introduction-of-the-protein-digestibility-corrected-amino-acid-score-method/51E5092761DA6004F1B081B204AAAB99
Posted by: petersonafess1946.blogspot.com

0 Response to "Which Of The Following Cooking Methods Would You Use To Increase The Digestibility Of Protein?"
Post a Comment